
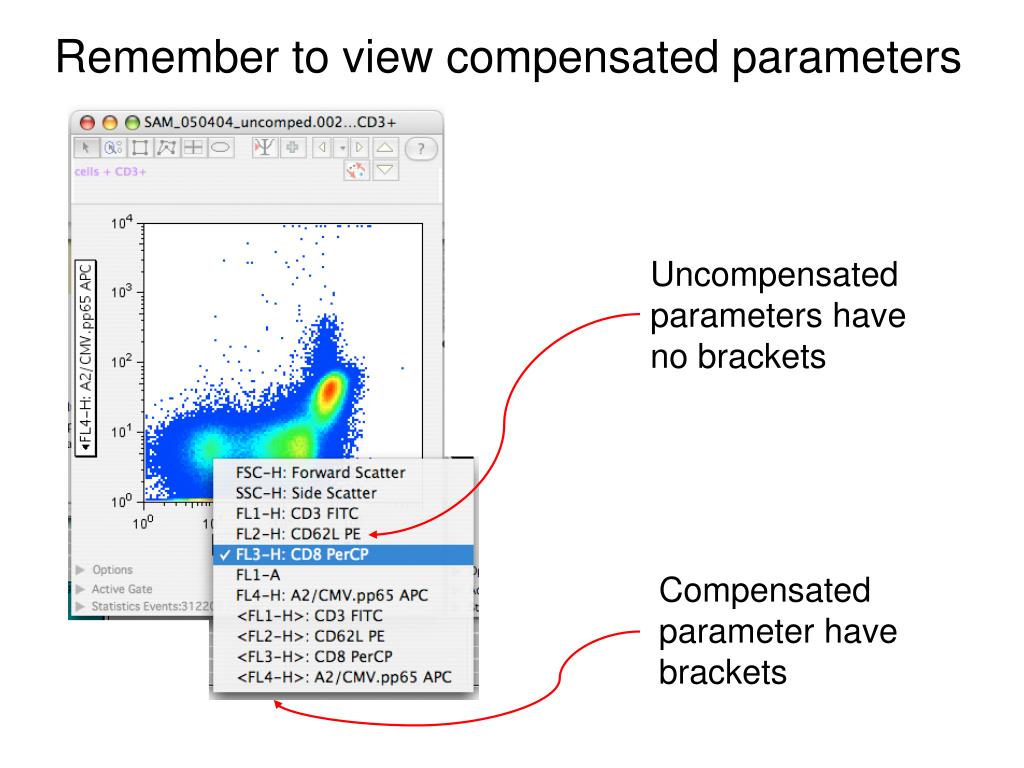
The compensation algorithm needs to be performed with a positive population and a negative population. Tandem dyes from different vendors or different batches must be treated like separate dyes, and a separate single-stained control should be used for each because the amount of spillover may be different for each of these dyes. Note: Although it would seem safe to assume that all tandem dyes created with the same donor and acceptor would have the same emission, this is not the case. The exceptions to this are tandem dyes, which cannot be substituted. Antibody capture beads can be substituted for cells and one fluorophore conjugated antibody for another, as long as the fluorescence measured is brighter for the control. The staining of the compensation control must be as bright as or brighter than the sample. The important rules to remember when using single stained samples for compensation are: This spectral overlap should be compensated for every fluorophore used. Figure 21b shows how the data looks when properly compensated. Single staining will reveal the level of spectral overlap between different fluorophores and allow you to remove or compensate for this overlap ( Chapter 2, Figure 12). This can be seen in Figure 21a where the fluorescence of FITC can be detected in the PE channel. Since compensation is a statistical calculation, the more data collected, the more accurate the compensation will be.Īs shown in this data below, as the number of collected events increases, the compensation values move towards the actual compensation value.Single Staining and Compensation ControlsĪs mentioned in Chapter 2 when performing multicolor flow cytometry, single stained samples are essential to determine the levels of compensation. Step 2: Collect the data and make sure there is a sufficient number of events.Īfter staining the carrier, it’s time to collect the compensation controls. Often times, staining the beads with 1/2 to 1/10 the concentration used on the cells will keep the signal on-scale, while keeping the signal above that of the cells that the compensation is to be applied to. Instead, simply re-stain the beads with less antibody. When this happens, do NOT turn down the voltage to bring the signal on-scale. Very often, compensation beads are stained with too much antibody and as a result, the fluorescent signal goes off-scale. Conversely, the amount of antibody the beads are stained with is less critical. The biggest concern with preparing proper compensation controls is that the fluorescence intensity of the controls must be at least as bright as that of the cells that the compensation will be applied to. However, beads cannot be used for some dyes, like viability dyes (such as PI, 7AAD, DAPI), fluorescent proteins, and other protein reporters (redox dyes, JC1, Ca++ dyes). Autofluorescence is not a factor since all the beads have the same autofluorescence values.Clear positive and negative signals show up on your control plots.This results in the brightest signal possible for your controls. All the antigen is captured in your solutions, not just some of it.Cells are not wasted when preparing your compensation controls.Using beads offers several advantages for compensation, including… The choice of the carrier is up to you, but for antibodies, the use of compensation beads is strongly recommended. The role of the carrier is to bring the fluorochrome to the laser intercept point. Choose the correct carrier for compensation.Ĭompensation is a property of the fluorochrome you’re using in your experiments. (If you’re interested in following along with this blog, you can find the data used in this experiment at this link here.
#Compensation flowjo software
The best practices for compensation involve following some very specific rules.These best practices also involve the use of automatic compensation protocols that are available in all major data analysis software packages. 4 Steps To Compensating A 4-Color Experiment Without knowing the median fluorescent intensity of the positives in the negative channel, or being able to evaluate the spread of the data, it is impossible to determine which of these above plots display the properly compensated values.


 0 kommentar(er)
0 kommentar(er)
